Drivers Qrs Diagnostic
VectraCor brings full-function spirometry to off-the-shelf computers and tablets.
Smart
ASUS Support Center helps you to downloads Drivers, Manuals, Firmware, Software; find FAQ and Troubleshooting. Orbit™ Portable Spirometer - Full-function portable spirometer which connects directly to your PC, laptop or tablet - Simplifies spirometry by eliminating calibration and sterilization with disposable nose-clips and pre-calibrated mouthpieces - Integrates with Office Medic software to analyze and customize spirometry data and reports - Meets the ATS/ERS 2005 Standard, British Thoracic.
PC based system that connects with major EMRs
Efficient
Silicon portals driver download windows 10. Pre-calibrated mouth pieces
Accurate
Comes with GLI-2012 predictors
Reliable
3-year warranty included

Product Number
Z-7000-0101
Office Medic Software
Office Medic is easy-to-use software that ships with all QRS ECGs and spirometers. Office Medic allows you to acquire, store and review diagnostic data using an off-the-shelf laptop, desktop or tablet.
About:
- Office Medic instantly stores the test data in its central database allowing you to manage and review it from anywhere.
- Create customizable reports in PDF, JPEG and TIFF formats to integrate with your EMR.
- Language Options: English, French, German, Italian, Portuguese, Spanish and Japanese.
Orbit Brochure (French) |
FAQs – Spirometry |
| Orbit System Requirements |
High-Filtration Efficiency. Tested specifically with the Orbit™ Spirometer Pre-Calibrated Mouthpieces. The VB Filter for Orbit™ Spirometer provides a high degree of protection for the patient as well as your testing equipment, while its low resistance allows for accurate testing results.
Independent laboratory tests have verified that this pulmonary function filter is highly effective. The VB Filter for Orbit™ Spirometer consistently filters out more than 99.999% of bacteria and 99.99% of viruses. At the same time, The VB Filter for Orbit™ Spirometer easily surpasses published ATS recommendations for flow resistance in pulmonary function instrumentation, which suggest resistance should be below 1.5 cm H2O/L/sec at flow rates of 14 L/sec.
Drivers Qrs Diagnostics
• Bacterial filtration efficiency: > 99.999%
• Viral filtration efficiency: > 99.99%
• Resistance to flow:
• 0.75 cm H2O L•sec-1 @ 14 L•sec-1
• 0.66 cm H2O L•sec-1 @ 12 L•sec-1
• Exceeds ATS and ERS standards
• Membrane protects electrostatic filter media
• Effective dead space: 45 ml
• For single-patient, single use only
• Free of latex, PVC, DEHP, BPA
All consumables assist in the accurate and comprehensive diagnostic testing performed with VectraCor devices.

Product #: Z-5000-2608
These mouthpieces are individually calibrated during production using the same equipment LDS Hospital uses to validate spirometers against the American Thoracic Society’s Recommendations for the Standardization of Spirometry. These pneumotachs are single patient use only.
Product #: 724050-00
QRS, in line with ATS Guidelines, recommends the use of nose-clips when performing a Spirometry test with a QRS spirometer.
Product #: Z-7000-2032
Pressure tubes connect the pre-calibrated mouthpieces to the QRS spirometers. They are re-usable, but need to be replaced if kinked or if condensation forms inside the tube.
Pressure tubes measure 48” long.
Product #: 723000-00
QRS sells a three liter Volume Calibration Syringe designed to fit the QRS spirometers. Although QRS spirometers cannot be calibrated in the field, the syringe allows you to check your calibration. According to ATS Guidelines, the volume accuracy of the spirometer must be checked at least daily.
QRS Diagnostic (National Biomedical LLC)
QRS Diagnostic, LLC (National Biomedical LLC) is an ambulatory cardiac monitoring company that designs and develops their own ECG products. National Biomedical LLC was founded in 1994-1995 and is headquartered in Maple Grove, Minnesota. The company specializes Holter Monitoring and Cardiac Event Monitoring technologies including ECG software analysis & management of the ECG data.
QRS Diagnostic also provides a patient information management system called the ‘Office Medic’. This patient management system allows users to acquire, store, and review diagnostic ECG data using laptop, desktop, or mobile tablet. QRS Diagnostic offers its products through a domestic network as well as overseas.
QRS Diagnostic takes a unique stance to offer high quality & cost-effective Holter Monitoring devices available, Through continuous development and innovation, QRS Diagnostic complies with all applicable regulatory and manufacturing requirements such as ISO 13485 Registered Quality Management System and are CE marked in accordance with MDD 93/42/EEC.
Competitive Advantage in Holter Monitoring
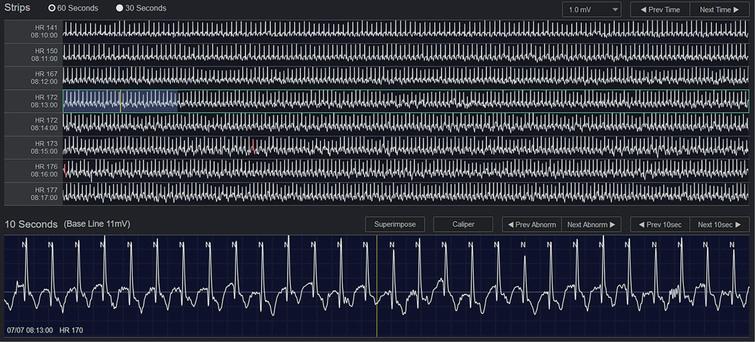
QRS Diagnostic clearly markets their products and services as a high quality solution at a fraction of the cost of other Holter Monitoring products. According to QRS, their Holter Monitoring devices & software are estimated at ’25 percent to 50 percent less’ than traditional Holter Monitoring devices. In addition to being a lower cost provider, QRS products are known to be diverse in function with expedited analysis capabilities. QRS Diagnostic has recently introduced a new multi-function Holter / Cardiac Event Recorder called the Q200/HE. This device a combination of a 24 hour – 14-day Holter monitor plus functions as a 30-day event recorder. The Q200/HE interfaces with the QRS Holter LX Analysis software to quickly & accurately capture transient cardiac events with flexible reporting capabilities.
Holter LX Analysis Software provides varying levels & capabilities based on the needs of each customer. The Q200/HE interfaces with Holter LX Analysis to view, interpret, analyze and report the ECG data. The Holter LX Analysis software is available in several languages including French, Spanish, Portuguese, Italian, Polish, German and Turkish languages.
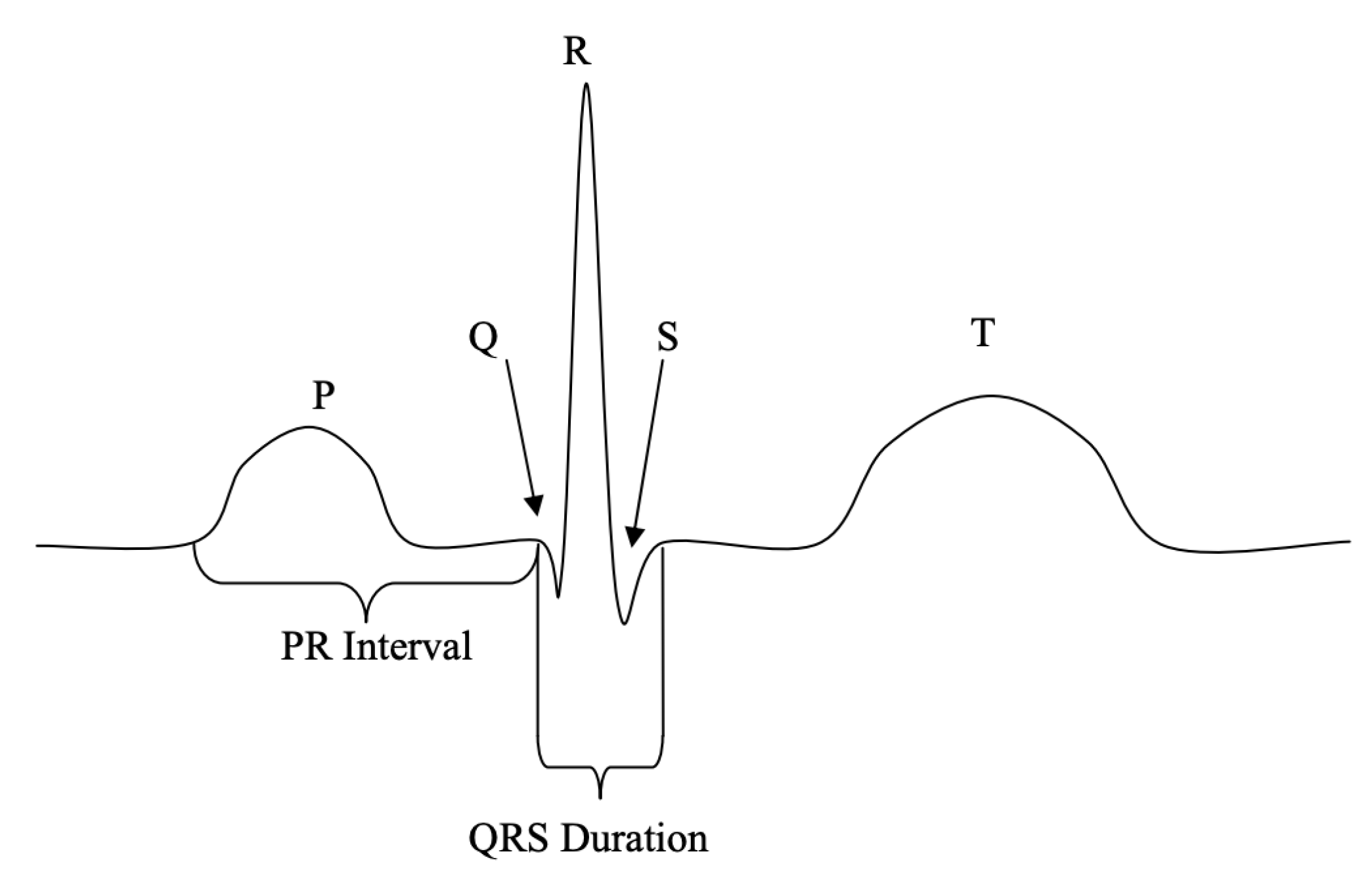
For more information, click QRS Diagnostic Holter Monitors & Software.
Drivers Qrs Diagnostic Tools
Legal & Safety Notice
Drivers Qrs Diagnostic Test
QRS Diagnostic has a sound reputation for manufacturing products with all of the safety accreditations including ISO & CE® mark. There is no public notices regarding QRS Diagnostic Holter Monitoring products